Fibre, Root & Horn | 2:
What the functions and neuropathologies of the dorsal horn, dorsal columnar and spinothalamic tracts reveal about the course of human nature and its evolution.
The Dorsal Horn
The cerebral tissues of the human brain consist of white matter fibres and grey matter in the cortical (outermost) layer of each hemisphere. Inferiorly, this configuration inverts in the inferior medulla of the brainstem, with grey matter interior and white exterior as it descends, and becomes continuous with the cervical spinal cord levels C1 to C8, and throughout the spinal cord until the grey matter tapers as the conus medullaris.
The spinal cord mediates an array of sensations which are crucial for survival and relation to the outside world, itself containing tracts of grey and white matter that are interlinked and compartmentalised with precision signals, each transmitted by dedicated fibres and codified appropriately so they may be conveyed to and from the brain / periphery to provide information.
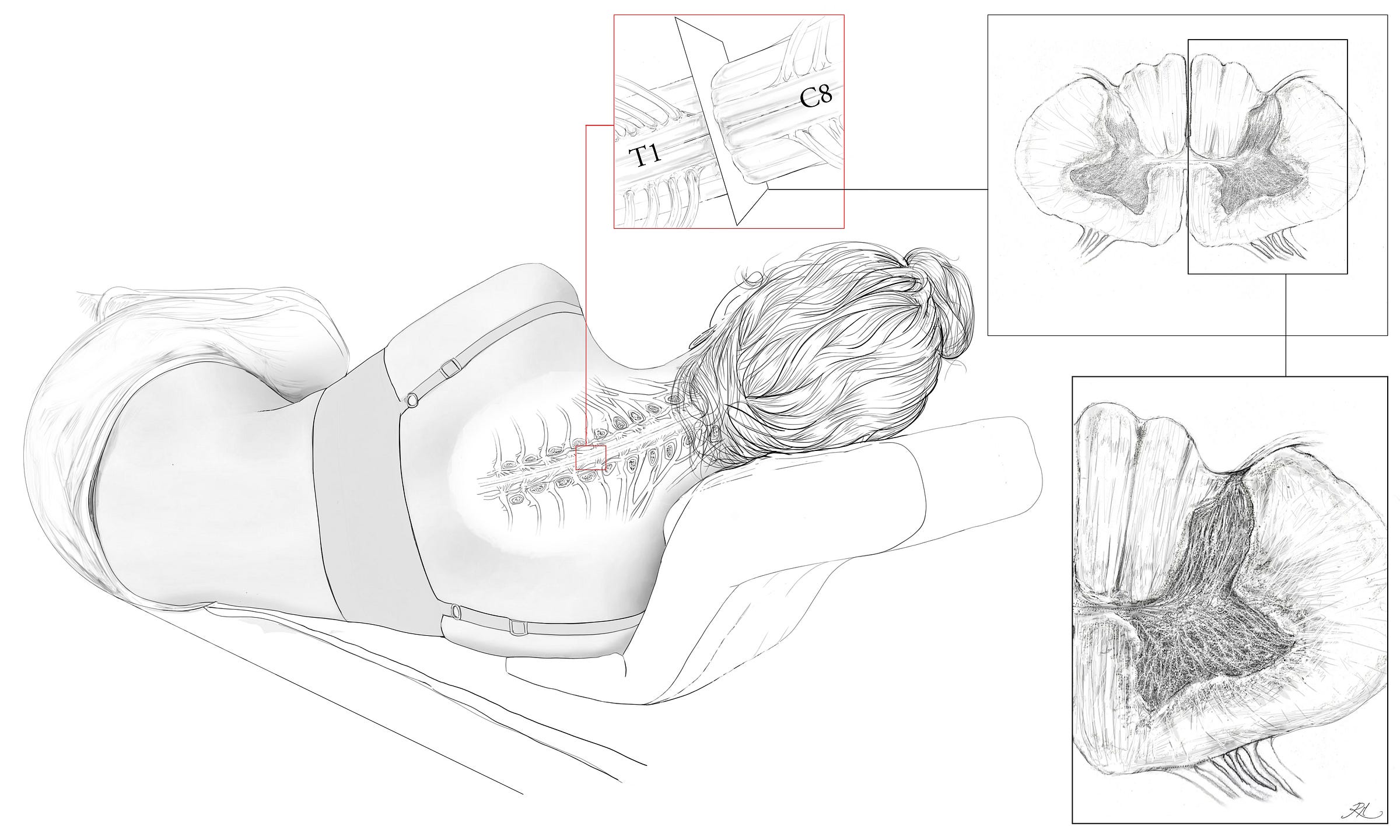
Within the spinal cord grey matter, the upper half of the butterfly like shape is referred to as the dorsal horn, and it is in this tissue that a variety of 1st order nerve fibres synapse from outside the spinal cord with a vast array of interneurons. The interneurons are fundamental to how physical pain is gated within the spinal cord, as they relay codified signals into 2nd order fibres, exciting or inhibiting ascent to the supraspinal pathways (the brain/stem), thus determining if the pain becomes conscious.
This LV article will focus on the nerves and tracts of the spinal cord in terms of sensation and how losses and gains in neuronal structure can cause nociceptive and peripheral neuropathies.
On Pain
‘I can't tell you how I knew - but I did know that I had crossed The border. Everything I loved was lost But no aorta could report regret. A sun of rubber was convulsed and set; And blood-black nothingness began to spin A system of cells interlinked within Cells interlinked within cells interlinked Within one stem. And dreadfully distinct Against the dark, a tall white fountain played’. ~ Vladimr Vladimirovich Nabokov.
Nabokov’s verse implies the experience of a heart attack, using visceral language to evoke a sense of sudden dread and vascular trauma. In terms of neural sensation, experience of pain within the viscera is led by interoceptive sensation, and due to the chemical signalling and structure of specific C-fibres which codify this pain, tending by default to have a more dull, burning and poorly localised codification when compared to exteroceptive sensations we experience. Visceral pain quite often will refer sensation, inducing ‘referred pain’, to the dermatome (the network of sensory nerves in the skin) or myotome (in the muscle).
Referred pain is induced by the convergence of cutaneous with visceral afferent nociceptive fibres onto the same (ascending) 2nd order neuron, via interneuronal synapsing in the laminae of the dorsal horn. We experience this as either sharp or dull pain referred from the organs or muscles of the torso, due to the distribution of fibres in the visceral and parietal peritonea (the membrane lining the abdominal cavity) of the abdomen. The pain alluded to in the poetic example above alludes to the effect of cardiovascular nociception, which incidentally can manifest as referred pain from vagal nerves which innervate the teeth and lower jaw, but is more often present as pain in the chest (via T1 to T4 sympathetic nerves) and numbness or pain in the left arm due to loss of circulation.
To understand these different fibres and how they shape the reality of our sensations relative to the extrinsic world on a more daily basis, as well as in pathophysiology, we must first understand each sensation as an adaptive function. For this article we will look mainly at peripheral rather than visceral sensation.
Anatomical terms and labels are key indicators of structure, function and location. Neurologists and neuroscientists use the terms nociception, thermoception and pruriception to denote physical pain, thermal sensation and itch respectively. The crucial distinction between these terms is that they delineate sensations which each confer a survival benefit, unlike pathological sensation, which confers no benefit and instead is an affliction. These discrete sensations also indicate the organisation of nervous tissues and their development across natural history, largely in response to change in the external environment, but also to perceive the state of oneself, as bodily internal tissues (the interoception aforementioned).
Sensation and affect are also worth delineating. Pain includes nociception, but also assumes a range of sensations which can be more affective, coinciding with emotion. In terms of clinical neuroanatomy, the limbic system of the brain can itself induce nociception in conditions such as somatisation, where mental anguish brings about physical pain, fatigue and discomfort.
Structures of Sensation: Nerve Fibres
As humans, we intuit from experience that nociception is not absolutely equivalent to ‘pain’. For something to be a sensation of pain, it is the nervous transduction of a noxious (harmful) stimulus, that is, the transdermal action of a pin perforating the skin damages / causes chemical attack of the free nerve endings of primary afferent fibres (the 1st order nerves of peripheral sensation) calibrated and oriented to detect this damage, largely classified as Aδ and C-fibres.
We can specify the functions of each so that their differences are clear. In the broadest terms, there are two types of sensory receptors in the skin, the mechanical and the chemical. Thermoreceptors respond to thermodynamic states, which is a little more nuanced, but part of chemistry.
The Aδ fibres are myelinated, 1-5 micrometres in diameter and project as mechanically sensitive free nerve endings into the epidermal skin cell layer, and in circumference around various hair follicles in the dermal layer (1), relaying signals at a speed of 5 to 30 m/s.
The Aδ nociceptive fibres are all reliant on peptides to chemically signal that something sharp has come into contact with the skin or that a hair follicle has been damaged; but the free nerve endings which are in the epidermis of both glabrous and haired skin are high-threshold, requiring a noxious stimulus well beyond the low-threshold fibres that detect the movement of both villus and terminal hairs.
All nociceptors respond to stimuli at a high enough threshold to ensure they are only activated if tissue damage occurs, and Aδ fibres by necessity relay highly localised and sharp nociception to the spinal cord as one complete 1st order neuron, conducting the signal via the dorsal root ganglion. This ensures an instantaneous aversion to pain through the sensation of sharp objects that can damage cutaneous and subcutaneous tissues.
The C-fibres are unmyelinated, 0.2-1.5 micrometres in diameter, and are slower by a factor of 10 or 15 ( at 0.5 to 2.0 m/s). Some use peptides to mediate chemical signals (called peptidergic fibres) and some do not.
Peptidergic C-fibres induce a dull, persistent ache, lasting beyond any initial stimulation of Aδ fibres (see Table 1.). They are calibrated to chemically receptive sensation, having evolved to transduce an array of signals via discriminative receptors. These include ATP receptors (the energy molecule adenosine triphosphate is inherent to all cells and is released during cellular trauma) and TRPV1 (Transient Receptor Vanniloid 1), which, even if its name does not roll off the tongue, does reside within it, codifying heat through the increased acidity caused by inflammation trauma. This includes the capsaicins, which evolved initially among certain plants to deter predators that present a cost of energy to the plant. The TRPV1 receptors can detect noxious levels of heat directly, but the binding of capsaicins to the active site of the receptor stimulates the same thermo-nociceptive pathways in mammals. As in human neuroanatomy, nature conserves adaptive functions across different stimuli, allowing for both clinical and lived inferences to be made because of the consistency of these stimuli in the context of everyday life.
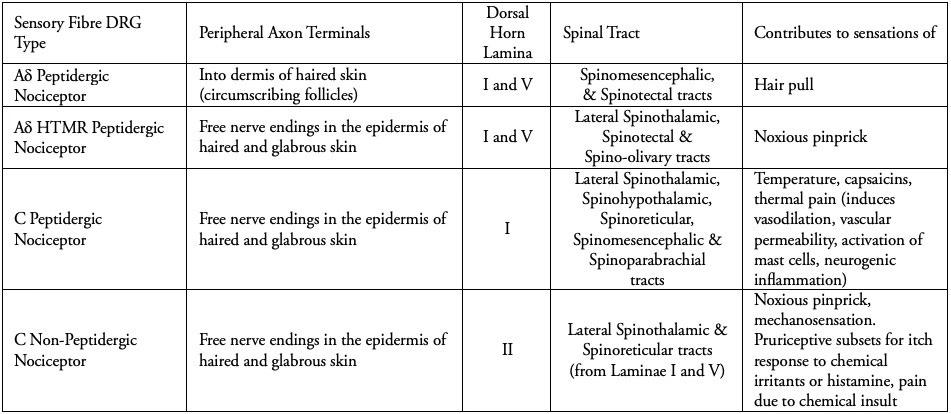
As tabulated above, C-fibres provide us with a reminder of the event which caused tissue damage, alongside a codified sensation, memorable enough to improve association and avoidance of the stimulus in future. A cut to the skin feels like a sharp and very defined pain (A-delta fibres), whereas there may be a general pain in the area which persists that is burning (nociceptive C fibres). A bruise tends to be the result of deeper tissue damage, and so results in a dull burning ache, but may also include abrasion of the skin. The neurotransmitter glutamate is responsible for mediating the high-speed, sharp, well localised pain, lasting for only a few milliseconds via peptidergic A-delta fibres. In contrast the neurotransmitter coined as, 'Substance P’ (the pain substance) is released slowly and builds in concentration, which induces the slow chronic pain we experience following an injury.
The lower velocity of nerve conduction in nociceptive C-fibres relative to Aδ reinforces the functional role of this reminder pain, as nerve conduction velocity is most important in terms of preventing contact with noxious stimuli from entering the skin, as the most peripheral tissue layer, and thus causing a physical withdrawal from the stimulus that is reflexive rather than conscious. Hence most organisms have their own reflex arc, which in humans enters via the dorsal horn and proceeds directly through the ventral horn to elicit a nocifensive (withdrawal, jumping, contracture) motor response. This can also take the form of immobilisation, where we perceive pain from proprioceptive sensory organs, and are prevented from moving by either reflex or conscious pain, in order to avoid further damage by our movements.
Once the damage has occurred however, nociceptive pain plays a crucial role in initiating the immunological and inflammatory responses that precede healing. This for example is why non-steroidal anti-inflammatory drugs (NSAIDS) can inhibit healing, especially of tendons and ligaments, as they block the enzymatic production of prostaglandins that cause inflammation. This inflammation also initiates the cellular signalling cascades crucial for repair. Inflammatory pain is also crucial in that it lowers the nociceptive threshold significantly, making any tactile or mechanical stimulation to an inflamed area much more painful in order to prevent literal insult to injury.
The 1st order sensory neurons which provide sensation by synapsing in the spinal cord are unlike neurons of the central nervous system (protected by the blood-brain barrier) in that they are readily exposed to the extravasation of substances through the endothelium of fenestrated capillaries. Capillaries are microvascular, and have evolved these fenestrations (‘little windows’ if taken literally from the original latin) in order to allow gases to diffuse via their walls. This means that circulating inflammatory signalling molecules, drugs and toxins can make contact with these first order sensory neurons via interstitial diffusion from capillary beds of the deep, papillary and superficial layers of the skin, eliciting physical pain we associate with its injury. In terms of human evolution, and the complex nutrients, drugs, and analgesic behaviours which we have the capacity to engage in, these mechanisms have allowed us to survive, grow larger brains, and mitigate pain, as well as expand pleasure and evolve bonding mechanisms which are socially and biologically complex. We can see this evolution in terms of the chemical, mechanical and tactile sensory organs themselves, and the unique tract which evolved to allow their signalling of the brain.
The Elder & Younger Tracts
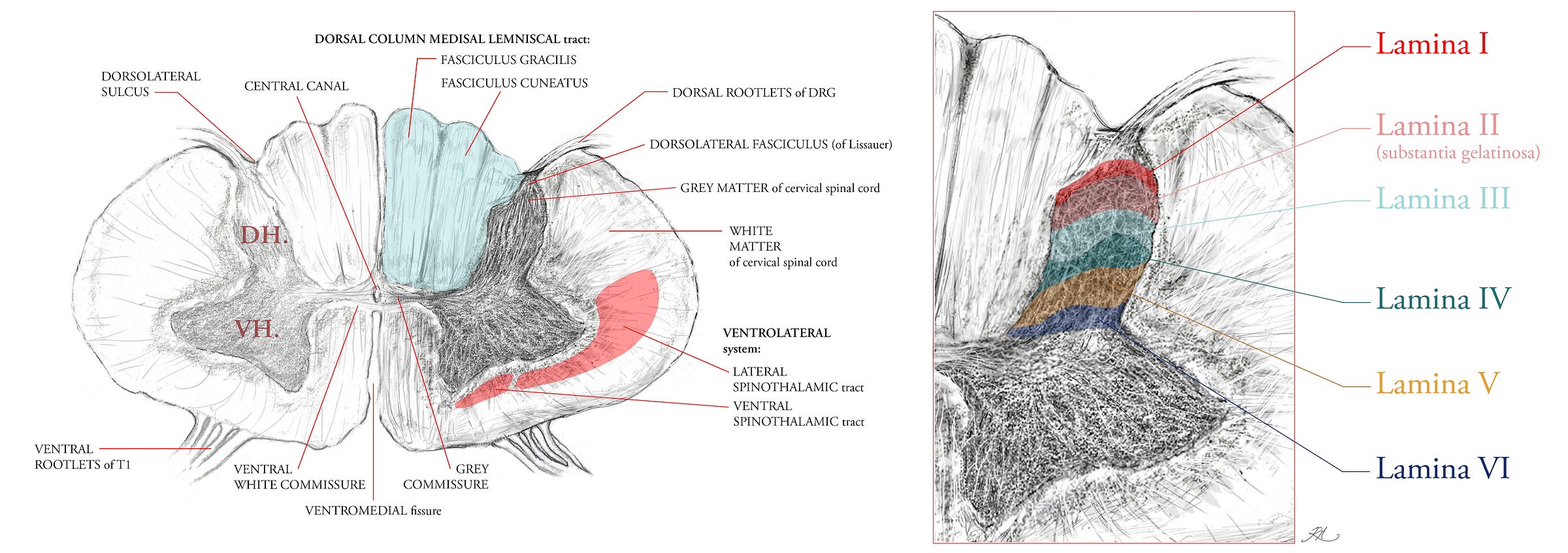
The Dorsal Column Medial Lemniscal tract was discussed in the previous article, mentioning its key functions and that it evolved far later in vertebrates than the sensations of the Ventrolateral (anterior lateral) System. It is composed of two columns (tracts), the fasciculus gracilis (the tract of Goll) and the fasciculus cuneatus (the tract of Bardach), which carry sensory input from the lower and upper body respectively. The columns are positioned in the dorsal half of the spinal cord, hence the name dorsal column, with the medial lemniscal tract forming from the decussation (crossing over) of 2nd order ascending internal arcuate (‘arching’) fibres in the medulla, which then synapse with a 3rd order neuron in the thalamus. The DCML thus takes its name from the ascending synaptic path of these fibres into the somatosensory gyrus of the cerebrum, the outer cortical layer of the brain, where each area of the body is topographically represented across the grey matter of this gyrus.
The distribution of ascending spinothalamic fibres (part of the Ventrolateral System) indicates its earlier evolution within nervous system, with only 10 to 25% of its fibres ascending as 2nd order neurons into the thalamus of the midbrain, the majority project into the mesencephalon, pons and medulla of the brainstem. These fibres first cross-over to the contralateral section of the spinal cord via the ventral white commissure (Figure 2), then in ascent, eventually project into the midbrain regions known as the tectal area and the periaqueductal grey (surrounding the aqueduct of Sylvius, and which circulates cerebrospinal fluid into the cord) because the fibres convey information about pain, temperature and crude touch which the brainstem and midbrain are configured to manage by analgesic response (enkephalins and endorphins).
The Spinoparabrachial and Spinomesencephalic tracts for example, are part of the Ventrolateral System along with the Spinothalamic tract, and respectively have aversion and analgesic roles relative to nociceptive stimuli, especially for the peptidergic C-fibres that detect thermally related pain.
Within the Ventrolateral System, the lateral spinothalamic area (Figure 2) predominantly contains the spinomesencephalic, spinotectal, and spinoparabrachial tracts.
In the ventral area of the spinothalamic tract reside the spinoreticular and spinohypothalamic tracts.
The spino-olivary and spinocerebellar tracts are not part of the Ventrolateral System, ascending their 2nd order fibres instead (respectively) in the dorsolateral (funiculi) white matter, and ventromedial white matter anterior to the ventral spinothalamic tract.
The lateral tracts is not as old as the ventral tract regions, and so the ventral is sometimes referred to as the paleo-spinothalamic tract, having a majority of C fibres configured to transmit crude touch and pain.
Both the DCML and VLS tracts are shown in cross-section of their fibre density area in Figure 2. Metabolically, the human central nervous system (brain and spinal cord) utilise 20 to 30% of the basal metabolic rate (caloric use at rest). The clinical symptoms of vitamin B12 deficiency reveal that the sensory fibres of the DCML are degenerated, by virtue of the fact that myelination is similar to the principle of insulating a wire, save that, nerve fibres are complex living cells that rely on saltatory (salt) conduction and ionic electrochemistry, not the electrical conduction of solid elemental metals. Myelin is itself a membrane rich layer, and all membranes are phospholipids.
The speed of nerve conduction is higher in some nerve fibres, with the fastest being those of proprioceptors in skeletal muscle at 80 - 120 m/s, with 13 - 20 micrometres of myelin in diameter (see A-alpha fibres in Table 2), which ensure muscles are not overstretched, and indicate to us the position of our limbs without visual confirmation. The next fastest are the mechanoreceptors of the skin, at 35 - 75 m/s, with 6 - 12 micrometres of myelin, and which include all of the A-beta low-threshold mechanoreceptors shown in Table 2.
When B12 becomes deficient, two production pathways necessary for myelination are affected: 1. is that lipid synthesis for incorporation into myelin by the enzyme methylmalonyl-CoA is not converted correctly, resulting in the abnormal accumulation of fatty acids in neuronal lipids, and 2. the hinderance of oligodendrocyte growth (the support cells which actually myelinate neuronal axons of the central nervous system), as B12 is a key biochemical step in DNA synthesis. Vitamin B12 deficiency can also be secondary to folate (vitamin B9) deficiency, or can induce microcytic anaemia (abnormally small red blood cells) due to its influence on protein synthesis and lipid incorporation (2). In terms of the evolution of the human nervous system, the requirement for B12 in sufficient quantities would have been essential for survival and reproductive capacity, allowing the dorsal column medial lemniscal tract and its sensory organ inputs to have evolved.
The skin itself is full of these sensory organs, each specialised with its own receptive (cutaneous) field, an area of skin yielding the response of a specific organ, and each its own elegant result of evolutionary research and development (in a manner of speaking). The low-threshold A-beta fibres (Table 2) innervate these organs and lanceolate (‘lance-like’) nerve endings in the epidermal, dermal and hypodermal layers. This is how vitamin B12 deficiency gradually induces paraesthesias, loss of vibratory sensation, and loss of proprioception.
A loss of vibratory sensation in this context indicates neurodegeneration in the DCML. Paraesthesias denote ‘any abnormal sensation, spontaneous or evoked, but not unduly painful or unpleasant’ (5), and if unpleasant are termed dysaesthesiae. If any pain is delayed following stimulation it can be distinguished as hyperpathia, which usually indicates that the spinal cord / supraspinal pathways have acquired an abnormal temporal and spacial summary of sensory signals, and that some form of neuropathic remodelling has occurred. These peripheral neuropathies can also be symptomatic of shingles, Lyme disease and diabetes.
If we look at Table 2, we can see each of these sensations is contributed by the 1st order afferent neurons listed. Their nerve endings and sensory organs transduce the stimulus into a signal, then into the spinal cord via the dorsolateral fasciculus (of Lissaur, Figure 2.), and synapse within the grey matter of the spinal cord before their signal ascends to the brain via 2nd order projection fibres in the white matter.
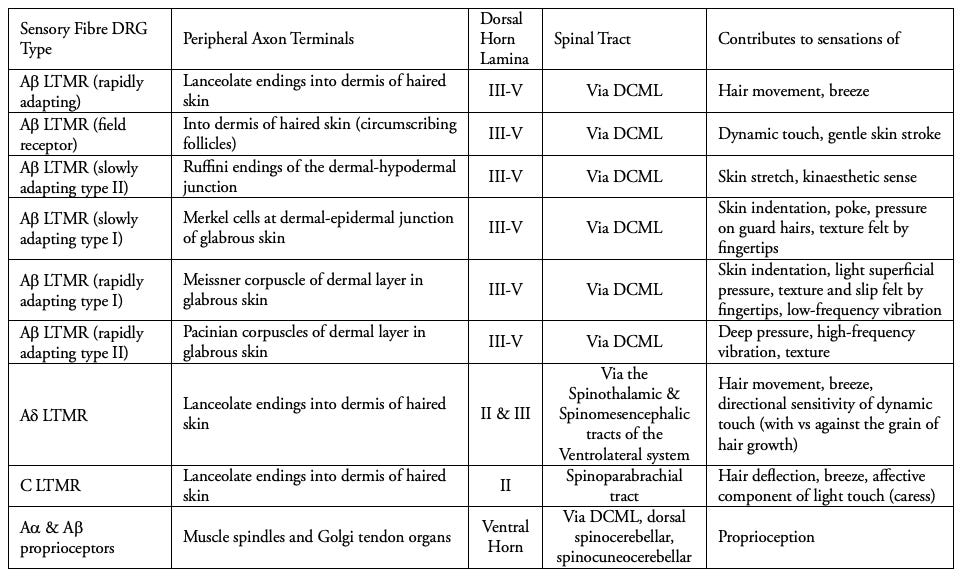
We see listed above the diverse fibres which conduct signals from these sensory organs, with their functions in the last column. The Ruffini nerve endings (sensing ‘kinaesthetic’ limb movement), Merkel cells (an adapted form of epithelial cell not derived from neurons, sense skin indentation) as well as the corpuscles of Meissner and of Pacini.
The C-fibre low-threshold mechanoreceptors are particularly interesting in terms of their evolutionary development and role in our relationships, for they transduce the sensation of hair deflection in a linear path across the skin, as the affective component of light touch (caress). Instead of spatial acuity, these fibres subserve tactile interactions in a receptive field of 1 to 10 centimetres, conducting at speeds of only 1 m/s. This relatively slow speed is conducive to sustained emotional touch as distinct from fast A-beta fibres and their discriminative sensation. Synapsing in lamina II, the afferent signal ascends via the spinoparabrachial tract, terminating in the insular cortex rather than the somatosensory, implying their role in interoceptive and emotional awareness. They are likely to have evolved, as they function similarly now, in order to codify slow-nurturing signals, promoting social cohesion, buffering of stress, and emotional regulation (3). Although they can also be stimulated under the right circumstances, by a gentle breeze.
The Islets of Gobel & Chronic Neuropathic Pain
Interneurons are grey matter intermediaries, which serve to either inhibit or excite signals between white matter nerve fibres. Because the majority of neurons in laminae I-III (Figure 2) have nerve axons which remain intrathecal (within the dura mater but outside the meninges, of the spinal cord) they are interneurons.
These axons arborise within the same lamina in which their soma (cell body) resides, but some fibre types cover a larger range of laminae within the dorsal horn. In murine models, A-beta and A-delta fibres span every lamina from I to V, respectively being more medial and lateral in their position in the dorsal horn. The peptidergic and non-peptidergic C-fibres are relegated only to laminae I and II (4).
With the exception of the C-LTMR fibres, which provide us with the aforementioned sensations that codify the caress experience, the distribution of the A fibres reflects their wide dynamic range; - a term used to denote that A-delta functions include a nociceptive range (Table 1), and A-beta provide an expansive range of non-nociceptive specialised sensations via the sensory organs and nerve endings (Table 2).
Inhibitory interneurons prevent signals from these fibres being transmitted to the brain, but the default distribution of fibres in the dorsal horn has been implicated as a contributing factor in neuropathic pain, wherein pathological arborisations may allow for signals to cross-talk between non-nociceptive and nociceptive fibres. The reality of neuropathogenesis in every neuropathic setting, from acquired physical trauma to inherited ion channelopathies, has revealed that there are numerous points of failure and need not be mutually exclusive. What is well established is that a changes in neuronal morphology chemical neurotransmission, and synaptic connectivity occur.
The Islets of Gobel are a type of interneuron residing in the deep half of II, with very significant responsibilities in the gating of nociceptive signals from the peripheral sensory organs and nerve endings of the skin. They utilise Gamma Amino Butyric Acid (GABA is the prevailing inhibitory neurotransmitter throughout the human nervous system) and / or glycine in order to propagate signals from one neuron to another.
The Islets have a wide spanning dendritic form which can ascend and descend longitudinally through up to 9 segments of the spinal cord, meaning that if their inhibitory functions are compromised, then the inhibition of pain at points of entry surveilled by this cell is reduced. Experimental evidence suggests that chronic neuropathic pain is not primarily the result of inhibitory interneuron cell death, although this is more so the case when contusive / ischaemic trauma to the spinal cord is involved. Instead the synthesis of the enzymatic precursor to GABA, Glutamic Acid Decarboxylase (GAD) has been shown to downregulate when 1st order afferent nerve signals to lamina II of the dorsal horn are compromised.
When nerve damage occurs, if secondary to a primary bodily trauma for example, the 1st order nerve signals which maintain inhibitory (GABA/Glycinergic) signals among the Islet cells can be diminished. This is a process where peripheral sensitisation leads to central sensitisation of the spinal cord, however, instances of spinal cord injury can lead to direct sensitisation of the spinal cord pathways without peripheral trauma. Ectopic sensitisation occurs when the abnormal arbors of a free nerve ending (see Table 1) can lead to arborisation of the fibres projecting from the soma of the nerve cell body into the dorsal horn lamina, arborising their also, and increasing synaptic connectivity with excitatory interneurons that function to propagate nociceptive signals into the Ventrolateral System (ascending pain tracts, Figure 2).
The complexity of response to injury within the axonal and nerve cell bodies of 1st order afferent fibres can also result from the invasion of afferent action potentials (the depolarisation of a nerve cell resulting in it ‘firing’ a signal) into other terminal branches of the efferent terminal axon, which can cause the release of neuroinflammatory substances (peptides for example), affecting both nearby neuronal and vascular tissues. Inflammation is a mechanism which has evolved to protect damaged tissues, but in neural cellular environments can also lead to abnormal functions with disproportionate effects on physiological functions.
Under similar conditions, the A-beta fibres we know the non-nociceptive functions of from Table 2 can gain access in some form to the superficial laminae I/II, see Figure 2), inducing allodynia.
Allodynia is a nociceptive pain evoked by a normally innocuous stimulus. The neurochemical and morphological changes to inhibitory and excitatory neurons are focal to the pathogenesis of allodynia, in which the functional distinction between innocuous and noxious signalling is compromised. Tactile allodynia is a mechanical form of the condition, as the loss of differentiation by a fibre type results in distinct symptoms of disease or injury. Most forms can result from central (spinal cord) sensitisation as primary injury, or peripheral sensitisation from primary injury (leading to central sensitisation as a secondary effect), the neuroanatomical crux is that each indicates an underlying difference in the nerve fibre types affected, and how the relevant tissues or neurochemistries have changed.
Persistent cutaneous mechanical allodynia often involves the wide dynamic range of A-beta fibres, but also the A-delta and C fibre types due to interneuronal circuit changes in the dorsal horn, and thus patients may experience pain in haired and glabrous skin as a result of nociception in subcutaneous tissues such as muscle. In tactile allodynia, abnormal nociception is induced by the A-beta low-threshold mechanoreceptive fibres, which are activated by various forms of light touch (Table 2).
The tragedy of chronic pain neuropathies is they recruit pathways which evolved for the adaptivity and specialised sensation of our species. It can be said of many diseases of course, that the tissues which have evolved as layers of many parts to make us human are all susceptible to disease. But for sensation to itself become the affliction marks a unique limitation on quality of life.
In either case, research has shown points of failure in hyperalgesic and allodynic states to be numerous. In central sensitisation of the dorsal horn, GABA interneurons can become less excitable due to the microglial (a support cell of the central nervous system) release of neuroinflammatory cytokines, which reduce their synaptic functions of pain inhibition. The function of ion channels can be disrupted, leading to abnormal anion gradients (differences in charge) which renders interneuronal dysfunction in lamina I. It has also been shown that a potassium cotrasporter (a kind of finely tuned toll gate in the membrane of interneuronal axons which exchanges potassium cations (+) and chloride anions (-) proportionately) can lead GABA / Glycinergic interneurons to change phenotype entirely, from being inhibitory interneurons to excitatory glutamatergic interneurons, inducing hyperalgesia.
Since the seminal initiatives of Ramón y Cajal at the turn of the 20th century, structure-function classifications have been attempted. Lamina I, through which the majority of nociceptive transmissions are relayed for either inhibition or ascent, contains fusiform, pyramidal and flattened cells, each having dendritic trees restricted to that lamina of the dorsal horn in order to isolate the codification of pain. The pyramidal cells for example, have (unsurprisingly) a pyramidal soma (cell-body) in all planes of section, and their arbors (dendrites) tend to remain in lamina I. There is a fourth class, the multipolar cells, which penetrate into the deeper laminae of the dorsal horn. If nothing else, the description of these differences in morphology reveal to us the sheer variety of interneuronal cells and their synaptic configurations, required to act by coordination of their circuitry and their timing, to maintain a clear and adaptive impression of the world within and the world without.
Lamina II, also known as the substantia gelatinosa (Figure 2) because of its low myelination, appears translucent in living tissue, and relays signals from C (caress) and A-delta LTMRs, and peptidergic (nociceptive) C fibres. In the remaining lamina, populations include the following key classes of interneuronal cell morphology: the central interneurons (elongated, similar to the Islets of Gobel, in the longitudinal axis), radial (with radiating dendrites through lamina II) and vertical (cone-like, with arbors through the dorso-ventral axis of lamina II).
Recent discoveries have shown that some of these interneuronal cell types are phasic, only depolarising into an action potential (conductive signal) as a phase; whereas others are tonic, firing slowly and continuously. It has been shown that in neuropathic states, pain signals are constantly active in the superficial laminae of the dorsal horn, with lowered inhibition by the responsivity of interneurons, like the Islets of Gobel.
Absent pathology, there is a constant balance between neurotransmissions made by inhibitory and excitatory interneurons, regulating the flow of codified signals into the supraspinal regions of the brainstem and brain. Pain, thermal sensation and crude touch came first in the lineage of vertebrate sensation, with more refined forms of sensation coming later.
Being able to experience and detect pain is however, crucial to what it means to be human and to our understanding of stimuli and hazards in the world.
Worth mentioning in closing, is the counter extreme to conditions of acquired pain, a condition known as Congenital Insensitivity to Pain Syndrome; a rare genetic disorder in which individuals are largely if not entirely anaesthesic in their cutaneous and subcutaneous tissues. The early development and survival of nociceptive fibres in this condition are compromised, and as such burns, cuts and fractures elicit no conscious reaction. Joints can become easily damaged, infections can go unnoticed, and on occasions where it develops in impoverished locations, at least one child with the disorder was known to perform an act to entertain. Some people considered the boy to be superhuman, blessed or immune to injury.
Understanding the nature of human sensation in both dysfunction and health, helps us to understand the underlying reality of phenomena we would otherwise deem strange. We better understand ourselves as a species, and our own individual experiences as part of a long continuum.
References:
Crawford, L.K. & Caterina, M.J., 2020. Functional anatomy of the sensory nervous system: Updates from the neuroscience bench. Toxicologic Pathology, 48(1), pp.174–189. https://doi.org/10.1177/0192623319869011
Al‑Chalabi, M., Reddy, V. & Alsalman, I., 2023. Neuroanatomy, Posterior Column (Dorsal Column). In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. Available at: https://www.ncbi.nlm.nih.gov/books/NBK507888/ [Accessed 2 Aug. 2025]
Marshall, A.G. & McGlone, F.P., 2020. Affective Touch: The Enigmatic Spinal Pathway of the C‑Tactile Afferent. Neuroscience Insights, 15, p.2633105520925072. doi:10.1177/2633105520925072
Li, L., Rutlin, M., Abraira, V.E., Cassidy, C., Kus, L., Gong, S., Jankowski, M.P., Luo, W., Heintz, N., Koerber, H.R., Woodbury, C.J. and Ginty, D.D., 2011. The functional organization of cutaneous low-threshold mechanosensory neurons. Cell, 147(7), pp.1615-1627.
Manji, H., Connolly, S., Kitchen, N., Lambert, C. & Mehta, A., 2014. Oxford Handbook of Neurology. 2nd ed. Oxford: Oxford University Press. p. 270.

